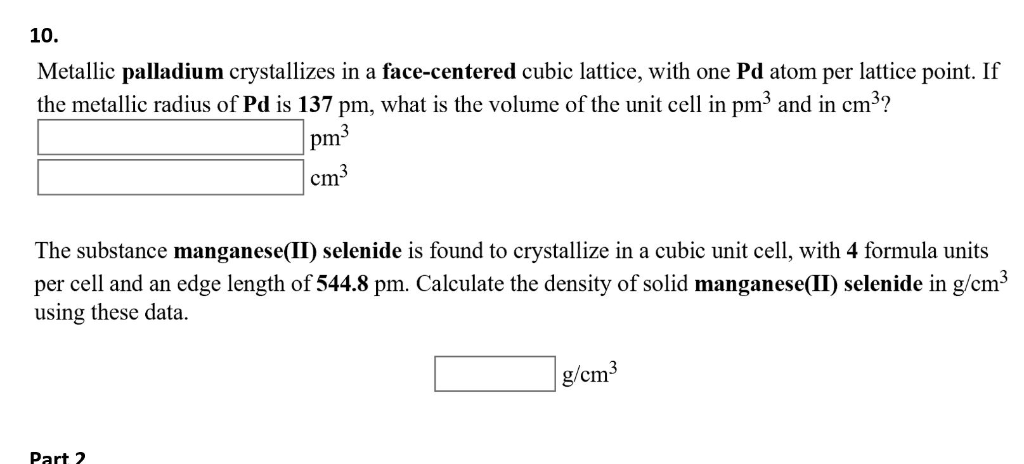
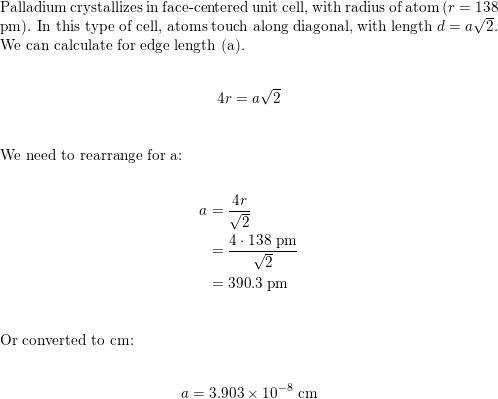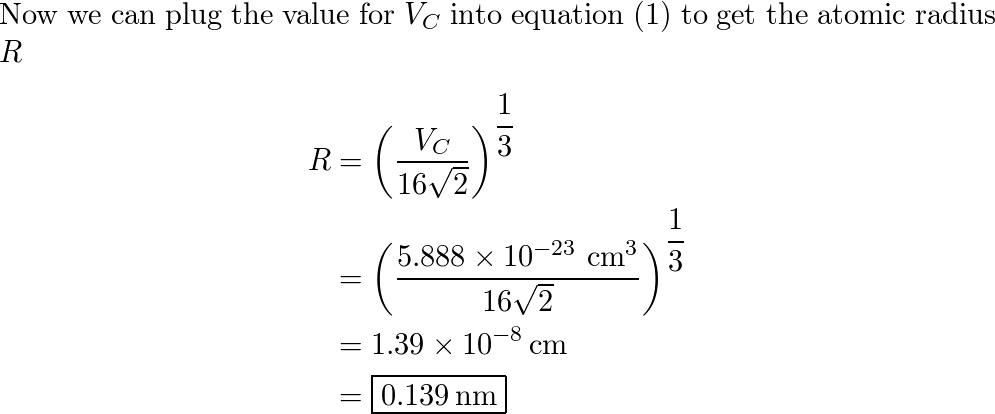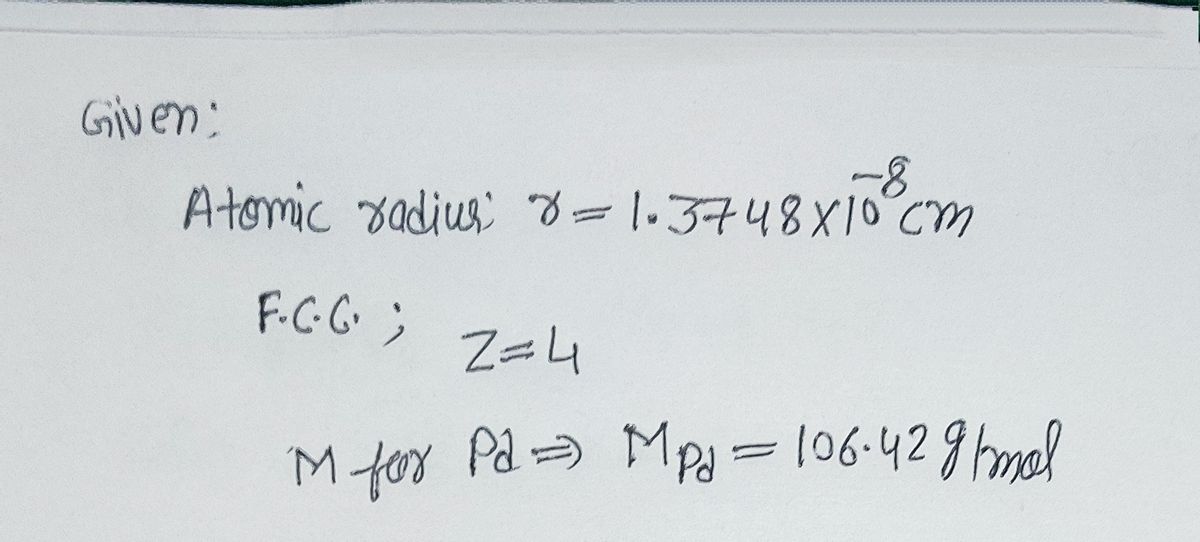SOLVED: Metallic palladium crystallizes in a face-centered cubic lattice, with one Pd atom per lattice point. If the metallic radius of Pd is 137 pm, what is the volume of the unit

Metallic lead crystallizes in a face-centred cubic lattice, with one Pb atom per lattice point. If the metallic radius of Pb is 175 pm, what is the volume of the unit cell

Chapter 3 Homework - Chapter 3 Homework Textbook Problems 9, 17, 22, 25, 33, 40, 44, 46, 71, 79 Additional problems and | Course Hero

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal

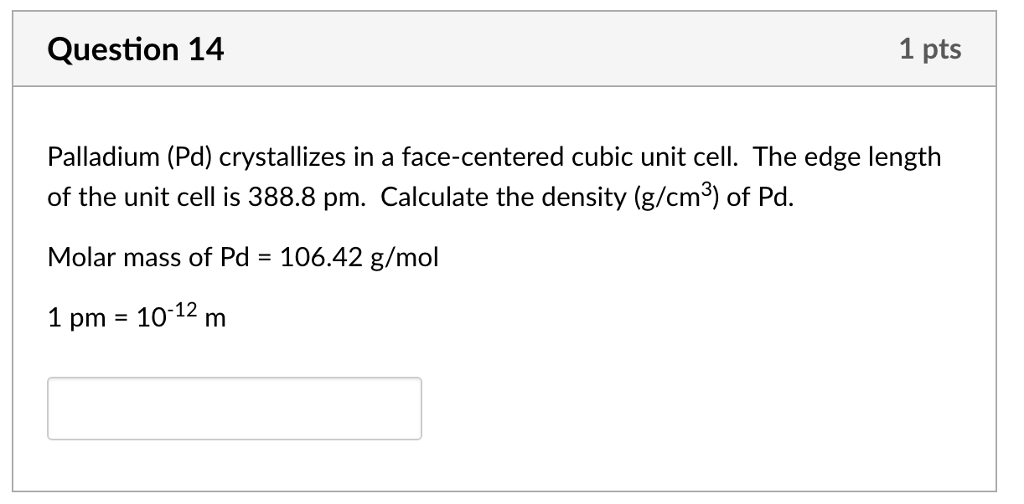
The metal palladium crystallizes in a face-centered cubic lattice with an edge length of 388.8 pm. What is the density of the palladium? | Homework.Study.com

Gold occurs as face centred cube and it has a density of 19.30 kg dm ^-3 .Calculate atomic radius of gold. (Molar mass of Au = 197 )
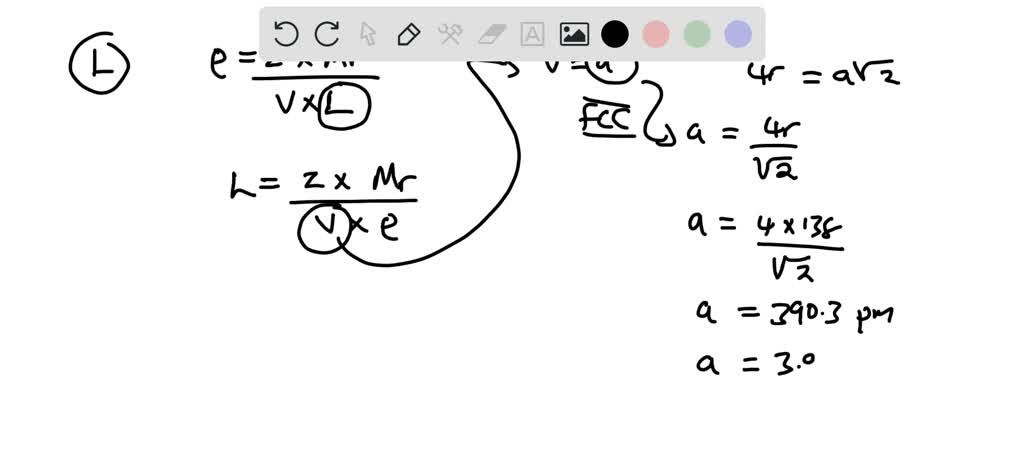
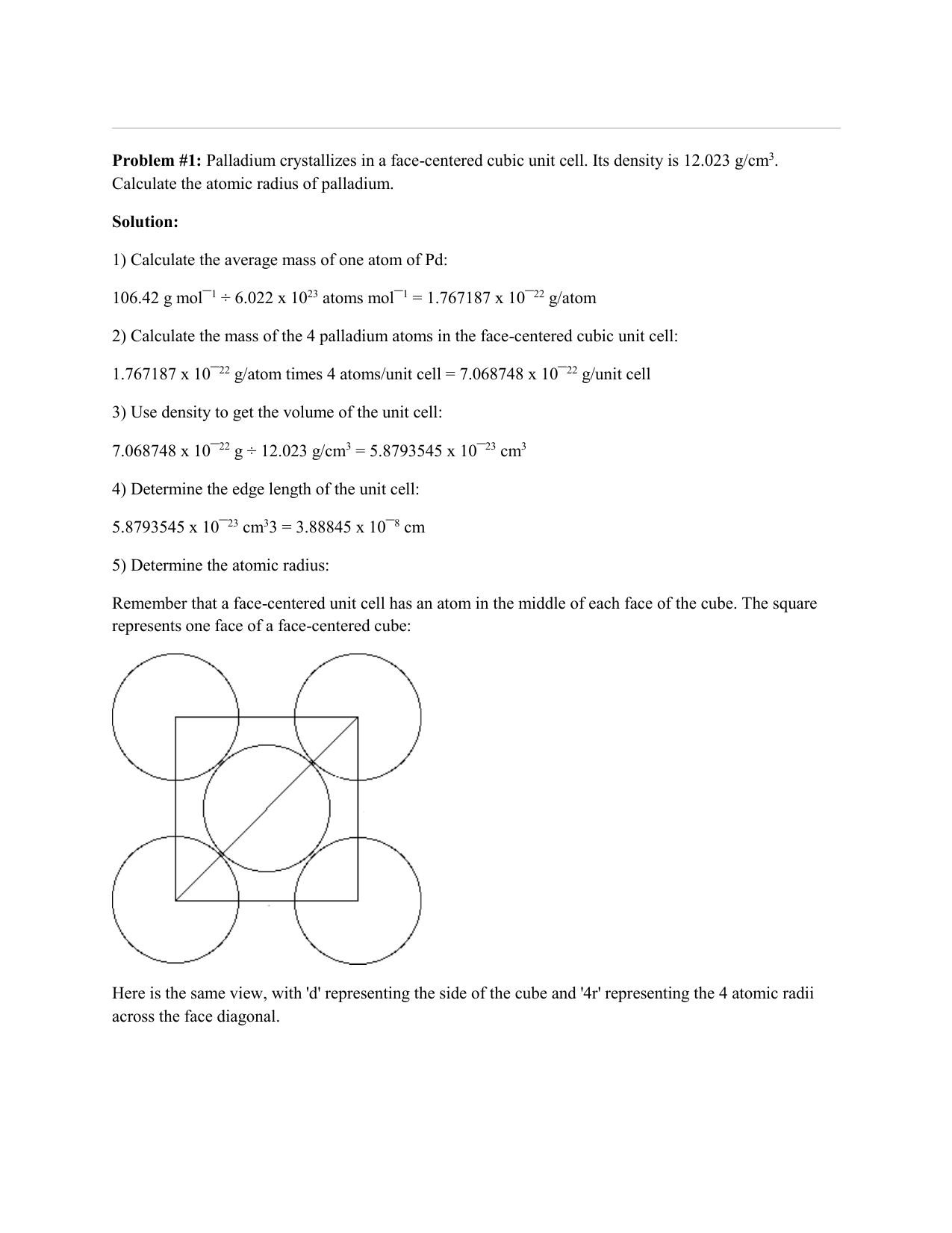
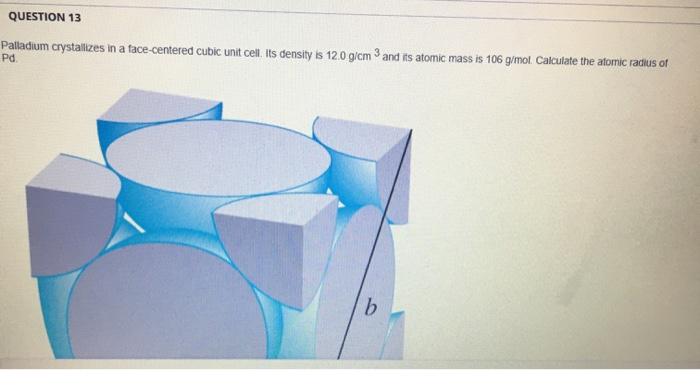
SOLVED: Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm^3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to estimate

OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...